Overview of PET in the Study
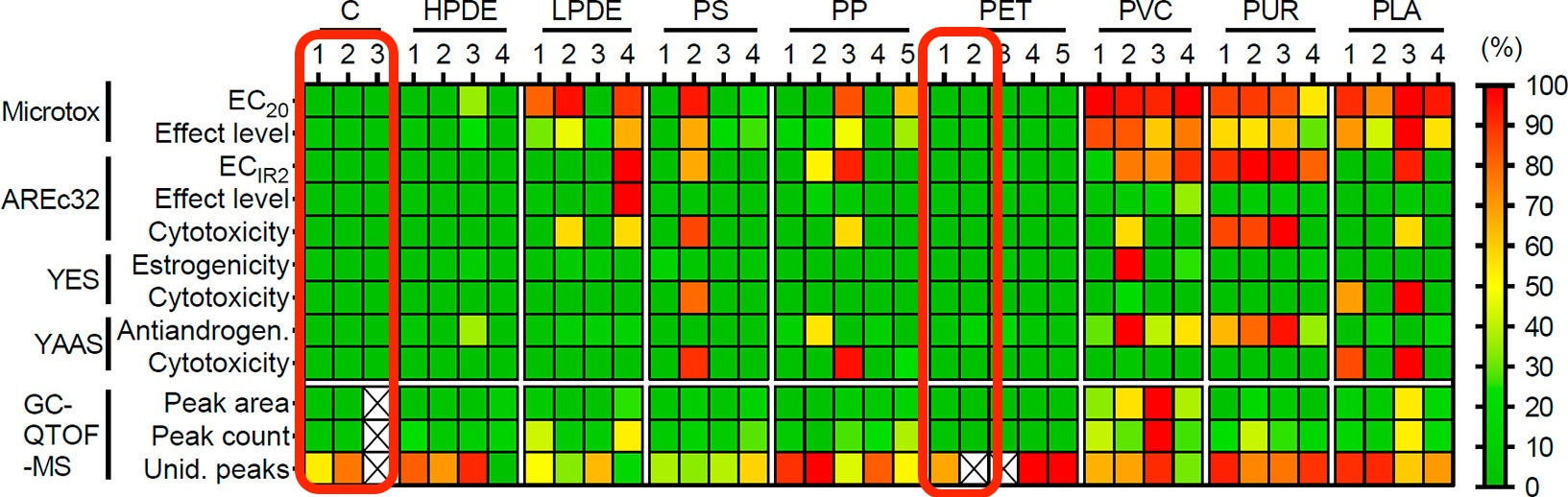
Polyethylene terephthalate (PET) is commonly used for food and beverage containers, such as water bottles and yogurt cups. In this study, PET was analyzed alongside seven other polymers to benchmark its toxicity and chemical composition. PET consistently emerged as the safest polymer, exhibiting no toxicity in the bioassays used.
Findings on PET
Toxicological Profile:
Baseline Toxicity: None of the PET samples exhibited baseline toxicity, as assessed by the Microtox assay, which measures bioluminescence inhibition of Aliivibrio fischeri. This absence indicates that PET does not release chemicals that cause nonspecific toxicity.
Oxidative Stress: PET had minimal activity in the AREc32 assay, which evaluates the induction of oxidative stress via the Nrf2-ARE pathway.
Endocrine Activity: PET extracts showed no estrogenic or antiandrogenic activity, unlike other polymers that triggered these effects. This indicates PET does not leach endocrine-disrupting chemicals.
Cytotoxicity: PET samples did not exhibit cytotoxic effects, either in human cells or yeast, further reinforcing its low toxicity profile.
Chemical Composition:
PET samples contained the lowest number of detected chemical features among all polymers, with a maximum of five features per sample in the GC-QTOF-MS analysis.
The chemical abundance (total peak area) of PET was also significantly lower than other polymers.
Comparison with Other Plastics
HDPE - A Low risk Polymer:
PET and high-density polyethylene (HDPE) exhibited the lowest toxicity across all assays. HDPE, like PET, demonstrated minimal baseline toxicity and no endocrine activity, making it another safer option among the tested polymers.
PET’s lack of oxidative stress induction was also similar to HDPE, reinforcing its position as a low-risk materials
Intermediate Toxicity Polymers:
Polypropylene (PP), and polystyrene (PS) showed variable toxicity depending on the specific product. Some items from these polymers triggered baseline toxicity or endocrine activity, while others were nontoxic, suggesting that their safety depends on the specific chemical additives used in production.
Toxic Polymers:
Polyvinyl Chloride (PVC): PVC was the most toxic polymer, with all samples showing high baseline toxicity and endocrine activity. PVC leached harmful chemicals like phthalates and flame retardants, which are known endocrine disruptors.
Polyurethane (PUR): Similar to PVC, PUR induced strong toxicity in most assays, particularly baseline toxicity and antiandrogenic activity. It also had a complex chemical profile with high chemical abundance and variety.
Polylactic Acid (PLA): Despite being a bioplastic marketed as an environmentally friendly alternative, PLA samples exhibited high baseline toxicity. This challenges its perceived safety relative to traditional plastics
Implications of the PET Findings
Advantages of PET:
PET’s low toxicity across multiple endpoints suggests that it may be one of the safest materials for applications such as food and beverage packaging.
Its low chemical complexity, with few identified hazardous additives or non-intentionally added substances (NIAS), further supports its safety.
Comparison with Bioplastics:
PET’s safety profile was notably better than that of PLA, a bioplastic often seen as a sustainable alternative. PLA’s baseline toxicity and more chemically complex profile indicate that it may not be inherently safer than traditional plastics.
Conclusion
The study highlights PET as a leading choice for safer plastic applications due to its low toxicity and chemical simplicity. In comparison, PVC and PUR demonstrated significant toxicity across multiple endpoints, while LDPE, PP, and PS exhibited variable results depending on specific products. These findings suggest that polymer type, combined with additive composition, heavily influences plastic safety. PET, along with HDPE, sets a benchmark for low-risk plastic materials.